Abstract
Background: Patients with classical Hodgkin lymphoma (cHL) relapsed/refractory to immunotherapies (e.g., brentuximab, anti-PD-1 antibodies) have poor outcome. We developed an academic CART30 (HSP-CAR30) targeting a proximal epitope within the CD30 molecule to overcome soluble CD30. We generated products enriched in memory T-cells to ensure persistence, and enhancement of antitumor efficacy (Alvarez-Fernandez et al, 2021). Here, we report the results of our Phase 1 study evaluating HSP-CAR30 for the treatment of R/R cHL and CD30+T-cell non-Hodgkin lymphoma (T-NHL) (NCT04653649).
Aim: Primary endpoints were to assess safety of HSP-CAR30 and to establish maximum tolerated dose (MTD) recommended for the following phase 2. Secondary objectives included response rates and cellular kinetics.
Methods: We conducted a phase 1 dose-escalation study in 11 patients with R/R HL or CD30+ T-NHL. cHL patients were R/R to treatments including chemotherapy, brentuximab, and anti-PD-1 antibodies, while T-NHL patients were R/R to at least 2 chemotherapy treatments. T-cells isolated from fresh leukapheresis of enrolled patients were transduced with a lentivirus encoding a second-generation 4-1BB-costimulated CAR, containing a scFv directed against an epitope within the proximal non-cleavable part of CD30 protein. Three cell-dose levels in three cohorts of patients (3+3 design) were evaluated: DL1 (3x106/kg), DL2 (5x106/kg) and DL3 (10x106/kg) CAR30+ T-cells.
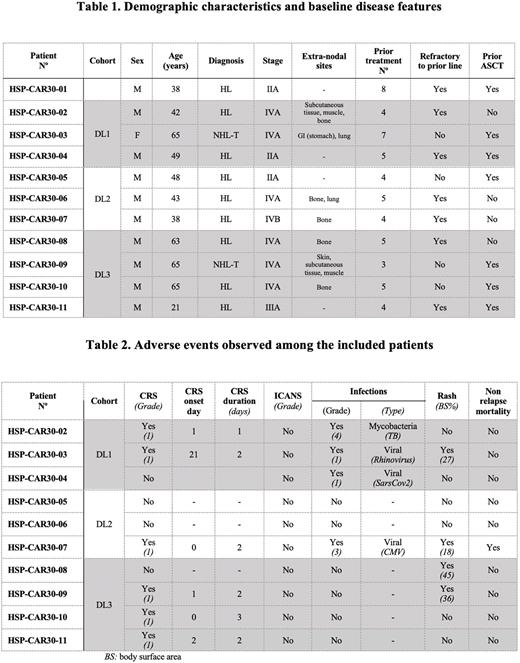
Results: From February 2021 to December 2021, 11 patients (9 cHL and 2 CD30+ T-NHL (1 ALK- ALCL and 1 PTCL NOS) were enrolled and underwent leukapheresis. Of these, 10 patients received HSP-CAR30: 3 patients at DL1, 3 at DL2 and 4 at DL3. Demographic characteristics and baseline disease features are summarized in Table 1. Median age was 50 years (range 21-65). Median number of prior lines of treatment was 5 (range 3-7). One patient was not infused due to lack of T-cell expansion. All patients received lymphodepletion before infusion: fludarabine/bendamustine for cHL patients (n=8) and fludarabine/cyclophosphamide for T-NHL (n=2).
Mean HSP-CAR30 expression of T-cell products was 94.79±1.07% (±SEM). Infusion products had a high proportion of CAR30+ memory T-cells: 26.09±6.06% memory stem (TSCM) and 67.52±6.07% central memory (TCM) in CD4+, while TSCM represented 42.2±5.4%, and TCM 50.5±5.7% of CD8+ CAR30 T-cells.
HSP-CAR30 was well tolerated; there were no dose limiting toxicities (DLTs). Relevant adverse events are shown in the Table 2. Cytokine release syndrome (CRS) was observed in 6 (60%) patients (all grade 1). No patient developed neurotoxicity. Self-limited skin rash was seen in 4 (40%) patients. One patient with history of cytomegalovirus (CMV) infections had CMV pneumonia. Another patient developed pulmonary tuberculosis. Long-lasting cytopenias (>3 months) occurred in 2 patients, with a complete recovery at 5 months in one of them. The other was diagnosed with myeloid neoplasm post-cytotoxic therapy (MN-pCT) with complex karyotype.
Best objective response was 100%, including 5 (50%) patients with complete response (CR), all with HL (DL1=1; DL2=3; DL3= 1). At data cutoff (July 25th, 2022), mean follow-up was 315 days (range 120-514) and mean PFS was 235 days (77- 444). All CRs were maintained during follow-up. Four patients died, 3 of them due to progressive disease (2 T-NHL and 1 cHL) and one with refractory MN-pCT while in CR of his cHL.
Mean time to HSP-CAR30 cell peak level across all doses was 21.5 days (range, 4-63). At the time of in vivo HSP-CAR30 expansion, memory T-cells (i.e., TSCM and TCM) were the dominant cell subset within CD4+ T-cells (TSCM 20.7±5% and TCM 56.33±6.2% of CAR30+ T-cells) and represented a high proportion of CD8+ T-cells (TSCM 18.36±4.9% and TCM 30.27±8.1% of CAR30+ T-cells). HSP-CAR30 was detected in blood samples by qPCR in all patients at 4 months and in all evaluable patients (n=7) at 9 months.
Conclusions: This is the first European academic CART clinical trial evaluating a T-cell memory-enriched CART 30. Our Phase 1 study provides evidence for feasibility and safety of HSP-CAR30. Additionally, HSP-CAR30 has shown promising efficacy in heavily treated cHL patient population, and this is being explored in the phase 2. Less-differentiated memory T-cells (TSCM and TCM) predominate at the time of in vivo HSP-CAR30 expansion. Updated clinical data and cellular kinetics studies will be presented at the meeting.
Disclosures
Caballero Gonzalez:Novartis, Gilead: Honoraria. Sierra:Jazz: Research Funding; Pfizer: Research Funding; Novartis: Honoraria. Briones:HOSPITAL SANTA CREU I SANT PAU: Current Employment; Celgene/BMS: Research Funding; Takeda: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Gilead: Consultancy; GSK: Consultancy; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal